
SERVICE
What is KMAP Express™?
KMAP Express™ is our dedicated research platform for understanding drug’s impact on cells on a genomic scale. We developed this technology specifically using our proprietary dataset, KMAP®. Our talented team empower scientists and researchers like yourself to make unexpected discoveries hidden behind the conventional knowledge. Please fill out the sample inquiry form to use our KMAP Express™ service.
Mode of Action
Up/down-regulated KMAP® Pathways and their corresponding genes can be inspected across samples using our web platform that is a visually informative and interactive user interface supporting instant searching, sorting, and selecting. Analytic results are conveniently presented in different formats like heatmap and plotting through our network viewer using state-of-the-art data visualization technology.
Target Deconvolution
Potential (off-)targets of a drug or drug candidate are inferred on the basis of expression similarity to drugs or compounds of known targets included in KMAP®. Multiple target prediction models are applied, and a ranked list of potential off-targets is provided based on the integrated score.
Matched Drugs
The expression profiles of disease models, whether cell, organoid, or patient, are evaluated against the extensive KMAP® dataset, indicating promising drug repositioning candidates. Your drugs of interest are compared against those of KMAP®, and the top-ranked KMAP® drugs are listed on the basis of expression similarity.
What is KMAP®?
Developed by KaiPharm, KMAP® is a high-quality NGS-generated full transcriptomic profiling dataset for thousands of small molecule drugs approved in the United States, the European Union, and Japan at three concentrations in two cell lines with triple replicates across all conditions. The transcriptomic profiles of your drug candidates are generated under identical conditions as those of KMAP® and comparatively analyzed against ~3,000 approved drugs (KMAP® version 2021), covering a broad range of targets and indications. In contrast to other assays or experimental approaches, the dimension of comparison in KMAP Express™ is unparalleled, interrogating 20,000 genes in 50,000 samples for each requested expression profile.
What will the next version of KMAP Express™ have to help my research?
We are excited to share that the next version of KMAP Express™ will include two major modules.
Side Effects
An inference module for side effects and toxicity is under development using AI driven approaches. Hundreds of side effects may be effectively predicted to guide your optimization steps in early drug discovery and development.
Matched Diseases
We have processed thousands of expression datasets and collected hundreds of disease signatures under strict quality control and data curation standards. By contrast-pattern matching between our disease signature dataset and your drug signatures, we can identify high-potential new indications to minimize your R&D efforts.
What if need more premium research?
Our primary mission of every partnership is to help you in the development of new and effective medicines, yet the approach to accomplish this goal will take different forms including long-term cooperation. Such partnerships are frequently critical to develop and optimize the synergies between what we provide and what you need toward success. Please call or submit a quote request to receive more information regarding our Premium Research Service, as the type of collaboration depends on the details of your project.
What are the advantages of KMAP Express™ than any other NGS Seq service?
KMAP Express™ is specialized for analyzing the ‘drug-induced’ transcriptome. From the perspective of a drug development, it aids in drug-design, the interpretation of results, and reaching conclusions. Furthermore, KMAP® is a large-scale drug-induced transcriptomic profiling dataset containing ~3,000 approved drugs to increase the chance of discovery compared with your compounds. By using KMAP®, you will objectively understand the base effects of your drug and determine what similarities it share with approved drugs from the United States, the European Union, and Japan. However, what is most important to us is your drug development process and goal. KaiPharm offers counseling services at least twice before setting up a detailed schedule and after confirming your final report.
Which sequencing platforms do you have?
We have Illumina and BGI NGS platforms, and we are currently setting up an automated library preparation system to increase our throughput.
How do I get the raw data?
Raw data and analyzed results are downloadable securely online through KMAP Express™
What kind of data and information do you deliver on the web platform?
We provide high-quality RNA Seq data (cloud storage or external hard drive available) as well as sequencing QC report, notation enriched functional pathways and inferred drug (off-)targets, and compiled lists of the identified DEGs and drug repositioning candidates.
PROCESS
What are the Workflow Stages in KMAP Express™?
Our KMAP Express™ workflow can be completed in less than 3 months, from the time of sample quality pass to analysis. There are six main steps after agreement with the experimental design, following consultations with KaiPharm. The status of your project, including all the relevant information for the process, can be securely viewed anytime through your account of our web platform. The main Workflow Stages are as follows:
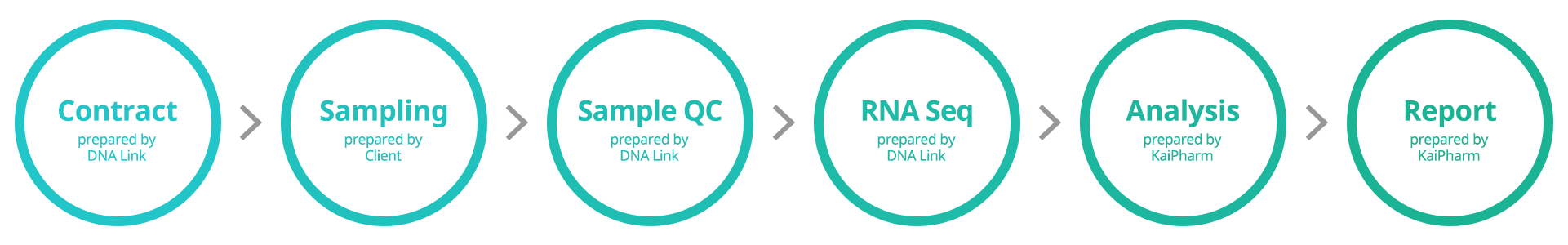
How do you address confidential information?
Before starting, the completion of a Non-Disclosure Agreement (NDA) will be recommended to protect any confidential information that you share with KaiPharm during your consultation.
How long is the process?
Once all tasks are scheduled, people are assigned to work on them. Normally, you can expect your KMAP Express™ Final Report within 3 months of the start date. More specifically, it usually takes 4 to 5 weeks for data generation after sample QC pass, followed by another 4 to 6 weeks for the analysis. However, in case of damaged samples, mechanical faults, unanticipated server issues, natural disasters, or other unexpected circumstances, we will not be able to guarantee the above schedule. Our team endeavors to ensure that your results finish on time.
What are your sample requirements?
For a drug of interest, the required amount or concentration will be provided at our first consultation. For RNA or Cells extracted from a disease model (e.g., cell, organoid, animal) after treatment, we require > 1μg of total RNA at a concentration between 15 and 20ng/μl or >106 cells shipped on dry ice. Please ensure that cells are washed with PBS to remove any culture medium before their collection by centrifugation. To provide tissues, we need a minimum of 5 g. If you intend to perform RNA extraction, please excise the tissue samples, perform RNA extraction, and then store the samples at -80°C. If RNA extraction is not being performed, please store the tissue samples immediately at -80°C.
How are sample packs shipped?
To use the KMAP Express™, the shipment of a small amount of your compound of interest is needed. Most projects include pick up services from DNA Link without extra charges.
What precautions should be considered in sample preparation?
Before sample preparation, it is recommended to share experimental protocols such as cell harvesting and RNA extraction to avoid miscommunication and ensure accurate transcriptomic profiling. Further, please pact the sample yourself ensuring it is packed well to prevent unwanted damage. For transcriptomic profiling (RNA Seq), please provide at least 48 samples. For drugs and biological samples like (RNA and Cells), they must be provided within a maximum of 5 days of collection to avoid degradation or contamination. You can send tissue samples/cell pellets using dry ice.
Intellectual property (IP) rights
Who owns any intellectual property rights arising from the results?
We do not claim any intellectual property. For advanced projects, we may claim license rights. All rights between parties will be defined and agreed upon in the contract.
How long will my project data be stored?
We store your samples and results of the analysis for 3 months after project completion. During this period, you can access the analysis report on the KMAP Express™ platform. Your data will be kept confidential and will not be shared with anyone else without your permission.
Who should I inquire about a project, including the sharing of confidential information?
An NDA is signed between you and KaiPharm before the start of any collaboration. Therefore, any information shared with KaiPharm to support your efforts is protected by the NDA as it is a legally binding contract. If you have any concerns or questions related to your project and the use of confidential information, you should consult with only KaiPharm’s representatives.
General
How do I pay? What are my options?
Payment of the price shall usually be paid on application for individual service results and by contract or Service Level Agreement terms as applicable. Payment can be made by credit/debit card on-line or over the phone, by EFT or by cheque.
I have a question not answered by this FAQ. How do I get it answered?
Please contact us by email (contact@kaipharm.com) or telephone (+82-2-3277-4294). It would be our pleasure to hear from you.
What is the role of KaiPharm and DNA Link in a project?
KaiPharm handles customer consultation, research design, and data analysis, and DNA Link is responsible for sample delivery and data generation as well as service payment.
What are the requirements of ID to create an account in KMAP Express™?
IDs are needs for several purposes, including user, project, and sample names. They must consist only of English letters, numbers, or” _” and be less in less than 30 letters in length.
What is the typical validity period of a quotation?
The validity of a quotation is 30 days from the date of issue.
Who are the designated representatives for my project?
Your designated team is composed of experts who have sufficient knowledge of biology and drug discovery to aid in your discovery efforts. If you login to the KMAP Express™ platform, you will see the designated team profile that supports your project.
